| @ |
[Preparation of sample solutions]5
Place 1 ml serum in a 10 ml stoppered glass test tube. Add 5 ml
acetonitrile, 0.5 g sodium chloride, 1 ml n-hexane and 25Êl
internal standard solution (4Êg/ml), and shake for 3 min. After
centrifuging the solution for 5 min at 3000 rpm, separate the
acetonitrile layer and distil under reduced pressure. Dissolve
the residue in 2 ml purified water, and extract two times with 5
ml and 3 ml n-hexane, respectively. Charge the Florisil-PSA
column with the n-hexane layer, wash with 3 ml n-hexane, and
elute PAE with 10 ml 5% acetone-n-hexane. Concentrate the eluent
under reduced pressure, and add n-hexane to 1 ml.
[Procedure for quantitative analysis]6
(i) Typical GC/MS conditions
Column: Fused silica capillary column, 0.25 mm i.d., 30 m long,
film thickness 0.25Êm; phenylmethylsilicone as the liquid phase7
Column temperature: 80, 3 min -> 10/min -> 300, 5 min
Carrier gas: Helium, total flow rate 50 ml/min, column flow rate
1.5 ml/min
Injection port temperature: 240
Injection mode: Splitless
Interface temperature: 300
Detection method: SIM
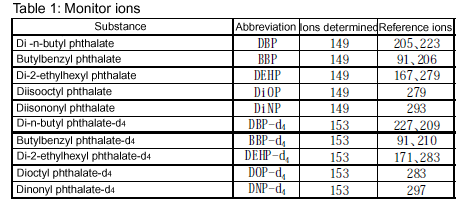
Monitor ions: See below.
 |
