| @ |
[Procedure for quantitative analysis]7
(i) Typical GC/MS conditions
Column: Fused silica capillary column, 0.25 mm i.d., 30 m long,
film thickness 0.25 Êm; phenylmethylsilicone as the
liquid phase8
Column temperature: 80, 3 min -> 10/min -> 300, 5 min
Carrier gas: Helium, total flow rate 50 ml/min, column flow rate
1.5 ml/min
Injection port temperature: 240
Injection mode: Splitless
Interface temperature: 300
Detection method: SIM
Monitor ions: See below.
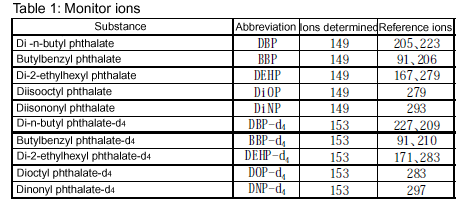
(ii) Quantitative analysis: Inject an appropriate amount of
the sample solution into the GC/MS. Check the peak areas for
each PAE divided by the peak area of the internal standard
against the calibration curve to obtain the concentrations of
the PAEs. Use the total area of the two major peaks for DiOP and
of the five major peaks for DiNP.
(iii) Calibration curve: Construct the calibration curve using
the PAE peak areas, obtained by injecting an appropriate amount
of the standard PAE solutions containing the internal standard,
divided by the peak area for the internal standard. Use the
total area of the two major peaks for DiOP and of the five major
peaks for DiNP.
@ |
