| |
(c) In vivo screening (iii)
In order to confirm the hormonal activities found in the in
silico and in vitro tests described above in the living body,
the uterine hypertorphy test for substances with estrogen-like
activities and the Hirschberger test for those with
androgen-like activities were performed. Analysis by the former
technique of 27 samples of existing chemicals and food-related
substances were completed, as well as the latter test for five
substances.3
In addition, an improved version of the OECD Test Guideline 407
(iterated administration for 28 days) was tested for the
effectiveness in screening with thyroid-related activities taken
into account.3
As in (ii) above, substances chosen for study for establishment
of guidelines and evaluation criteria (potential positives) are
those exist in the natural environment or the environment of
human life, for which any endocrinological effect has been
observed in wildlife or experimentally found. The screening
method for these substances should be capable of determining the
intensity of the effect with precisions sufficient for
elucidation of the mechanism of action. The methods that meet
this requirement are to be proposed for domestic and/or
international (pre-)validation, through which the guidelines
will be finalized.
(2) Priority list4
The priority list is being augmented by the results of the
screening tests described above.5
(3) Definitive studies
The methodology of the definitive studies should be improved to
overcome the limitations of the conventional multi-generation
reproductive toxicity tests. In response to this requirement,
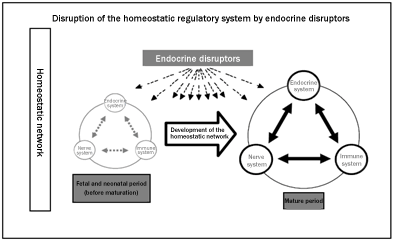
the lifelong test on rodents is under development, in which a
series of toxicity parameters (including those related to
disruption of the homeostatic regulatory systems, e.g. nerve,
behavior, and immune systems, and their maturation (see Figure),
beyond those used in the conventional multi-generation
reproductive tests) are evaluated comprehensively throughout the
life (including genesis, development, maturation and aging).
Exposure to bisphenol A during pregnancy and lactation was used
as the model in studies on nerve and behavioral systems.
Evaluation and mechanism of action of effects on behavior via
the dopamine and serotonin (5-HT) nerve systems, evaluation of
effects on higher nerve functions using operand conditioning of
mice, and analysis of effects on the sexual differentiation of
the brain belong to this category.
Click here to enlarge the image
|
