We provide prior consultation for applicants who submit application documentation for the designation of food additives and the revision of use standards.
JAPANESE Site![]() Back to Division of Food Additives,NIHS
Back to Division of Food Additives,NIHS![]()
2024-12-02updated

Flow of Consultation
The contents of the Website are amended in necessity and the revisions are occurred in Japanese site first. You may refer latest information in Japanese site.
| Consultation Process | |
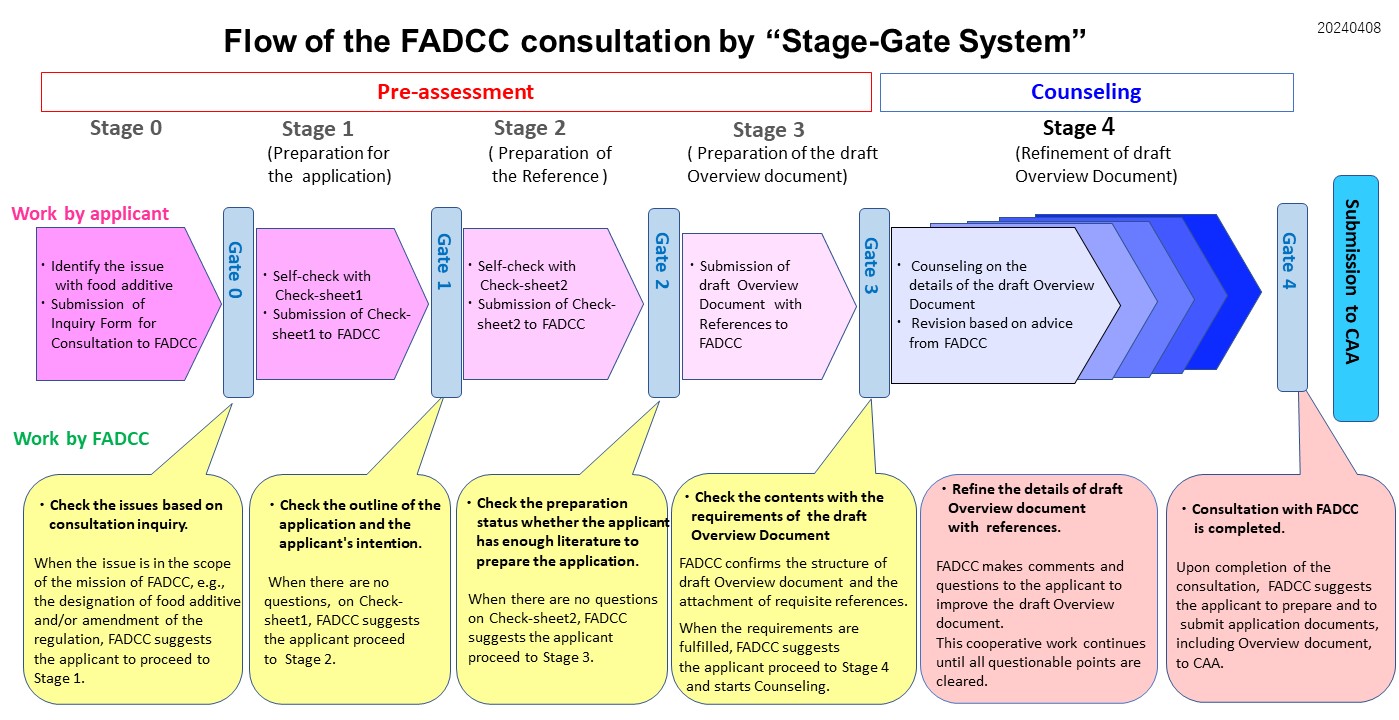
| Attention to readers The following describes the consultation process using the Stage-Gate System for non-native Japanese speakers.The system charts and texts on this website may be amended without further notice for the improvement of the system and/or web site quality. |
|
| [Pre-assessment] Stage 0 |
|
| [Organizing consultation content and preparing the Inquiry Form for Consultation] If you wish to initiate a consultation process with the FADCC, fill in the necessary items in the Inquiry Form for Consultation and send it to us by email. Refer to the Q&A for the FADCC consultation content. If the consultation content falls under FADCC's consultation services, the procedure passes Gate 0 and proceeds to Stage 1, where the FADCC requests the preparation of Check-sheet 1.*1 If it is not clear from the description in the Inquiry Form for Consultation that it falls within the scope of FADCC's work, we may ask you to provide additional information. Gate 0
[Review the Inquiry Form] The FADCC reviews the inquiry form and provides information on the case below which corresponds to your case. [1.Cases handled by the FADCC] The process passes Gate 0. The FADCC provides instructions forthe preparation Check-sheet 1*1, and you proceed to Stage 1. [2. Other cases] The FADCC directs the inquiry to a public health center or quarantine station, etc. |
|
*1: There are four types of Check-sheets, those for food additives in general,
food additives for fortification, enzymes, and flavoring substances.
Each involves a different form with different entry items
based on the type of substance in inquiry.
| Stage 1 (Preparation for the application) | |
|
[Collecting necessary information on the application substance] In Stage 1, using Check-sheet 1, you begin by checking the items to be addressed for preparation of the draft Overview document. The FADCC provides Check-sheet 1. Please, send it back to us via email after determining how much of the necessary information has been collected referring the Procedure*2 and Guidelines*3. *2: The Procedure for Preparing Application Documents for Designation of Food Additives and Revision of Use Standards for Food Additives. *3: Guidelines for the Risk Assessment of Food Additives (Revised Guideline for Assessment of the Effect of Food on Human Health Regarding Food Additives) Collect required information in Check-sheet 1, for all the requisite items.
Gate 1
[Confirmation of applicability of requests for designation, outline of the application and your intention (checking Check-sheet 1)] If the FADCC could confirm that the preparation for the application would be in progress, we would invite you for an interview. In the interview, the FADCC would receive your presentation on the proposal and would offer advice on preparing the draft Overview document for the application. The interview and the advice should be in Japanese. (You may accompany with own interpreter in these sessions.) If the preparation at this stage has been satisfactorily completed, the process passes Gate 1. The FADCC then provides Check-sheet 2, and the process proceeds to Stage 2. If Check-sheet 1 has elements requiring special address, the FADCC will instruct that the process should not proceed to Stage 2, but instead the Stage 1 application preparation status should be reviewed. If the safety information, among other things, is not complete, there is no hope of moving forward. |
|
| Stage 2 | |
|
[Preparation of references] Check the appropriateness of references with check-sheet 2 through writing outlines of references corresponding to each item. And send the Check-sheet 2 back to the FADCC via email after all the requisite items described.
Gate 2
[Checking the preparation status of references necessary for the application (checking Check-sheet 2)] If the FADCC could confirm that the preparation for the application would be in progress, we would invite you for an interview. In the interview, the FADCC would offer advice on preparing the draft Overview document for the application. The interview and the advice should be in Japanese. (You may accompany with own interpreter in these sessions.) If the preparation status for the references at this stage has been satisfactorily completed, the process passes Gate 2. The FADCC instructs you to begin preparation of the document and proceed to Stage 3. If Check-sheet 2 has elements requiring special address, the FADCC will instruct that the process should not proceed to Stage 3, but instead the Stage 2 application preparation status should be reviewed. |
|
| Stage 3 | |
| [Preparation of the draft Overview document] You prepares the draft Overview dDocument using the outline template provided by FADCC (see the bottom of this page), citing references. Procedure, Guidelines, and Handbook for the Procedure are helpful in preparing the document. When the document is prepared, send it with the references to the FADCC via email.
Gate 3
[Checking the requirements of the draft Overview document] The FADCC would confirm whether the document would be in the requisite structure according to the Procedure and the Guidelines, and whether the corresponding references would be attached. When the completion of the condition is confirmed, the process passes the Gate 3. The FADCC provides notice of receipt of the document to you, and the process proceeds to the document Counseling (Stage 4). If the document or references have points to be addressed, the FADCC will note and advise these, and you consider how they shall be addressed, correct the document, and request the FADCC to perform its check again. |
|
|
[The draft Overview document counseling] Stage 4 |
|
|
[Counseling on the details of the document and revision of the documents based on advice from the FADCC] At this point, focused counseling with the FADCC takes place. The FADCC checks the details of the document and sends comments to youf there are points to be addressed and corrected. You then consider how these should be addressed, correct the document, and request the FADCC to perform its check again. The above-mentioned checking and correction continues until the document reached the status to fulfill the requirement to submit the Prime Minister. The Consumer Affairs Agency (CAA) defines the appropriate specifications and conducts tests regarding these specifications. At the appropriate time, the CAA contacts you to request provision of samples of the proposed application substance.
Gate 4
[Confirmation of completeness of the responses to the Comments] When the FADCC has determined that no remaining points need to be addressed,the process passes Gate 4, and the FADCC instructs you to submit the requisite application materials, including the Overview document, to the CAA, and the consultation process ends by the FADCC. |
|
| [Materials used in the Stage-Gate System] | |
| [Check-sheet] The Check-sheets can be found in the following pdf files. The FADCC sends Word files to the applicant, which contain dedicated Check-sheets corresponding to the different application substances below. The pdf file below are sample Check-sheets. The FADCC will send the applicant Word files of the Check-sheets corresponding to the application substance. Stage 1/ Gate 1 Check-sheet1 for food additives in general sample Check-sheet1 for flavoring substances sample Check-sheet1 for enzymes sample Check-sheet1 for food additives for fortification sample Stage 2/ Gate 2 Check-sheet2 for food additives in general form Check-sheet2 for food additives in general -processing aids form Check-sheet2 for food additives in genera -breast milk substitutes form Check-sheet2 for flavoring substances form Check-sheet2 for enzymes form Check-sheet2 for food additives for fortification form 【Items to be included in the test report】 The following is a guide to items to be included in each test report. ・Items to be included in the validation report of purity and other test methods (Recovery tests) ・Items to be included in the Test Results report ・Items to be included in the validation report of analytical methods for food additives in foods (Recovery test) ・Items to be included in the test report for validation of the enzyme activity determination method Please refer to the Japanese version of each test report as well, as the Japanese version is the most up-to-date. Click here for the Japanese version. [Template of Overview document](in Japanese) When creating a draft Overview document in Stage 3, the FADCC will provide the applicant with a template that corresponds to the target substance. Template of Overview document for food additives in general Template of Overview document for flavoring substances Template of Overview document for enzymes Template of Overview document for food additives for fortification |
|