Contents
Organization Chart
Click to jump to each page.
History
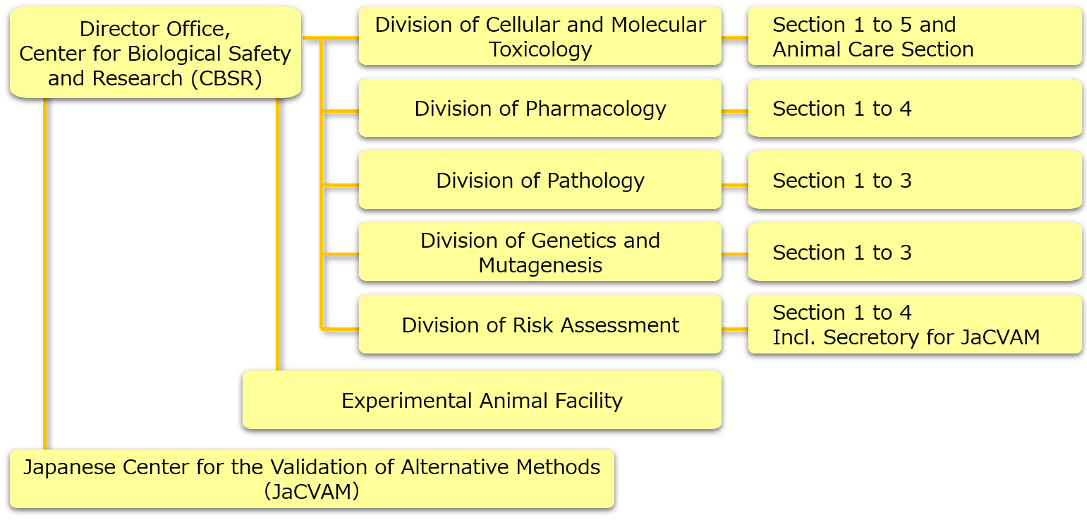
The CBSR (formerly known as Biological Safety Research Center, BSRC) of the NIHS was established on December 28, 1977 as the National Institute of Hygienic Sciences (Director: SHIMOMURA, Tsutomu) under a ministerial ordinance revision (enacted January 1, 1978, Minister for Health and Welfare: OZAWA, Tatsuo). Since the 1950s, various chemical substances have been introduced into our living environments (e.g., thalidomide, arsenic, methyl mercury, and PCB). As the effects of the toxicity of such substances on health started to garner attention throughout society, the CBSR was established for responding to such administrative demands. In addition to the transfer and new establishment of the Divisions of Cellular and Molecular Toxicology, Pharmacology, and Pathology, a Division of Genetics and Mutagenesis was newly established in April during the same year. In May, the CBSR was inaugurated as the only national risk assessment research facility in Japan with bioassay facilities (first CBSR director: IKEDA, Yoshio). Following subsequent organizational changes, the CBSR comprises five research divisions (Cellular and Molecular Toxicology, Pharmacology, Pathology, Genetics and Mutagenesis, and Risk Assessment) and an Experimental Animal Facility since 2015. Its mission is to research and test the safety of house-related substances such as medicinal products, cosmetics, foods, food additives, pesticides, industrial chemicals, and household products using biological resources (such as experimental animals and cells) and to conduct comprehensive safety assessments, including toxicity prediction methods based on scientific grounds.
Regarding safety testing for medicinal products, national testing of the pyrogenic properties of glucose injections and similar compounds was overseen by the Division of Pharmacology (before the inauguration of the CBSR, this was overseen by its predecessor, the Pharmacology Testing Division) from November 1950 through 1988. Additionally, the CBSR was involved in the introduction of standards related to conducting safety testing on medicinal products in Japan (Good laboratory practice [GLP]; notification issued on March 31, 1982) and establishing guidelines for testing methods to ensure safety of medicinal products in compliance with these standards. Until such duties were transferred to the Pharmaceuticals and Medical Devices Agency (PMDA) in 2014, a GLP Assessment Committee was established in the CBSR; the committee currently continues to cooperate in GLP inspections. The CBSR has also been involved in toxicity assessment for the approval and screening of new drugs. Even after the abolishment of the New Drug Approval Screening Investigating Committee in October 1999, it has continued cooperating by providing specialist committee members for internal screening at the Pharmaceuticals and Medical Devices Evaluation Center, the predecessor of PMDA.
The review of new chemical substances based on the Act on the Evaluation of Chemical Substances and Regulation of their Manufacture was first conducted by the Chemical Substance Safety Assessment Committee comprising the CBSR section chiefs and senior researchers. Second, the Chemical Substance Specialist Committee was involved in the evaluation process, which included division heads and external experts. Since 2003, deliberations have occurred based on the Act on the Evaluation of Chemical Substances and Regulation of their Manufacture by a trilateral council of the MHLW, the MOE, and the Ministry of Economy, Trade and Industry (METI). In 2009, the revised Act on the Evaluation of Chemical Substances and Regulation of their Manufacture was issued to realize “minimization of the effects of all chemical substances on humans and the environment by 2020,” which was the subject of the international agreement. To achieve this, Division of Risk Assessment Section 1 is taking the lead and CBSR offers full cooperation. Moreover, for safety inspection work on chemicals on the List of High Production Volume Chemicals of OECD, which began in 1990, the testing data assessment that the Health Bureau of the MHLW had outsourced to an external agency is assessed by the Toxicity Testing Investigative Committee and Chemical Substance International Safety Countermeasures Committee, which comprises experts from each division in CBSR, after which the results are reported to the OECD. The organization of data and preparation of testing plans for substances to be tested going forward have been conducted with the cooperation of each division in CBSR. These results are publicly available at our Japan Existing Chemical Database (JECDB) (https://dra4.nihs.go.jp/mhlw_data/jsp/SearchPageENG.jsp) and Japan Existing Chemical Toxicity Testing Report (1994–2006, vol. 1–13: issued by the Chemical Substance Inspection Promotion Liaison Council).
When the health-related effects of endocrine disruptors, such as dioxin, emerged as a social problem, the CBSR conducted basic research and became involved in the issue from a specialist standpoint for any related surveys, testing, and research. In 1999, when the MHLW and the MOE established a new tolerable daily intake (TDI) for dioxin, the CBSR contributed by assessing large amounts of toxicity data. Basic research has been conducted on the safety of various nanomaterials being produced with recent advances in nanotechnology. Such research resulted in establishing the grounds for classifying MWNT-7 as group 2B by the IARC (2015), which is a type of multi-walled carbon nanotube capable of causing similar damage to living organisms as asbestos.
The safety assessment of pesticides and pesticide residue was previously conducted in collaboration with members of the CBSR and the Division of Food, as well as external members of the universities and similar organizations. In 2003, this role was transferred to the Food Safety Commission established in the Cabinet Office. The CBSR personnel continue cooperating as specialist committee members on the Pesticide Specialist Investigative Committee Assessment Subcommittee of the Food Safety commission. Three years prior to the enactment of the positive list system for the pesticide residue regulations implemented in 2006, the CBSR personnel had been fulfilling investigation roles.
In particular, responding to issues related to the safety of food additives and the health effects of long-term exposure was an important issue directly leading to the establishment of the CBSR. The CBSR conducts testing and research related to carcinogenic substances, such as butylated hydroxyanisole, potassium bromate, and alizarine, and has provided scientific grounds for establishing subsequent administrative policies. Meanwhile, when the Food Sanitation Act (1947, law no. 233) was revised in 1995, the use of 489 additives included on the existing additive list (MHLW directive no. 120, 1996) continued to be recognized, leading to a revision of safety assessment. The Existing Natural Additive Safety Assessment Study (Group Leader: HAYASHI, Yuzo, fourth CBSR director) in the 1996 Health Science Research Report investigated the basic safety properties of these existing additives based on the results of international assessment, authorization status in Europe and United States, and the results of safety testing. Of the 489 additives, 139 required rapid confirmation of safety, including new toxicity testing; 150 did not require rapid safety testing at that time based on origin, manufacturing method, and properties; 159 had already undergone international assessment and had basic safety confirmation; and 41 did not require rapid confirmation of safety based on the obtained testing results. The repeated-dose toxicity studies (90-day repeated-dose toxicity studies) and genotoxicity testing (such as Ames test and chromosome abnormality testing) were successively implemented for 139 food additives requiring rapid and efficient safety confirmation and for the designated food additives classified a long time ago that required reevaluation for safety confirmation. The results obtained are evaluated by the Evaluation Committee hosted by the CBSR director and composed of the division heads of the CBSR and the Division of Food Additives, and experts from inside and outside the NIHS. As we have recently come close to reaching targets for this assessment, we decided to start reevaluating 150 food additives classified as “need not require immediate safety testing at this time based on origin, manufacturing method and properties” in the 1996 report. In fact, as 41 substances were later excluded from the list of existing food additives, we have established prioritization for the implementation of safety assessment of 109 substances and have begun a reassessment from 2017.
Furthermore, for respond to international trends for the promotion of the 3Rs (i.e., reduction, refinement, and replacement) of animal welfare in animal experiments that have been conducted to determine the danger (toxicity) of substances harming human health or the environment, the Japanese Center for the Validation of Alternative Methods (JaCVAM) (http://www.jacvam.jp/en/index.html) was established in 2005. The Director of CBSR also acts as the Director of the JaCVAM. JaCVAM established a secretariat position in the Section of New Testing Evaluation in the Division of Pharmacology (since 2015, organizational changes resulted in this being renamed the Section 2 of Division of Risk Assessment). In 2007, the Sixth World Congress on Alternatives and Animal Use in the Life Sciences (WC6) was hosted (Chairperson: OHNO, Yasuo, Director General/former Division Head of Pharmacology). In 2009, a memorandum of cooperation on International Cooperation on Alternative Test Methods (ICATM) was signed by Japan, the US, the EU, and Canada. In light of these initiatives, in 2011, the MHLW Evaluation and Licensing Division issued a communication on “Promoting the use of alternative animal testing methods and utilizing JaCVAM when preparing materials to apply for the authorization of quasi-drugs.” Based on this framework of international cooperation, the MHLW, other governmental ministries, industry bodies in fields such as cosmetics and chemical substances, universities, and academic societies are coordinating to contribute to the establishment of over 10 OECD testing method guidelines developed in Japan as well as proposing the results of many alternative assessment methods to governmental bodies for promoting the use of alternative methods in animal experiments.
The CBSR has also been currently participating in projects related to safety assessment by many international organizations (WHO, FAO, OECD, IARC, IPCS, and the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use; since October 2015, the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use [ICH]). In 1980, the NIHS participated as the leading Japanese organization in the IPCS planned by the WHO. Since then, the Division of Chemical Substance Information (currently, Section 3 of the Division of Risk Assessment) has played a major role in the provision of IPCS safety cards (ICSC; http://www.nihs.go.jp/ICSC/). At the ICH, which was established in April 1990, the CBSR have personnel participated in various expert meetings as a representative for the Japanese governmental agencies and have been involved in developing guidelines for nonclinical safety testing together with various experts in the EU, USA, and the world.
CBSR also participated in the Technical Cooperation Project of the National Medicinal Product Food Quality Control Testing Laboratory in the Republic of Indonesia and conducted personnel exchanges including technical cooperation and long-term business trips (1974–1989). In addition, exchange programs were conducted with overseas experts to exchange opinions and gather information related to chemical substance safety assessment based on the agreement between the Japanese and American governments on Cooperation in Research and Development in Science and Technology (Non-energy Division; theme: Toxicity: 1981–1998). During that time, CBSR held joint Japan–US conferences on establishing chemical substance risk assessments with the National Institute of Environmental Health Sciences (NIEHS) (held four times from 1981 to 1993). Furthermore, CBSR participated in the Tianjin Pharmaceutical Inspection Center Project (1994–1998) and the China Pharmaceutical Safety Assessment Center Project (1999–2006) by the Japan International Cooperation Agency (JICA). Moreover, CBSR held joint research workshops on regulatory science with National Institute of Toxicological Research of the Korean Food and Drug Administration (KFDA) (held three times from 2000 to 2006).
The research activities of each division of the CBSR have constantly played a central role, specifically in the field of toxicology. The CBSR was significantly involved in the development of this field and the establishment of the Japanese Society of Toxicology (former Japanese Toxicology Society; Chair at the time of name change: KUROKAWA, Yuji, fifth CBSR director/first director of the Japanese Society of Toxicology: OMORI, Yoshihito, second CBSR director), from a leading position. The CBSR is conducting basic research for providing scientific evidences prior to administrative responses to issues related to health problems by chemical substances in each era. These activities have been highly evaluated globally. In 1997, the CBSR was presented on the cover and cover legend of Cancer Research, a journal of the America Association for Cancer Research (vol. 57, No. 7; cancerres.aacrjournals.org/content/canres/57/7/local/front-matter.pdf) as a chemical carcinogenesis research facility. More recently, it has been proactively involved in introducing comprehensive gene expression analysis technology associated with whole genome sequencing into the field of toxicology. From 2001 to 2006, it created the basis for the current Toxicogenomics Project, including holding the Toxicogenomics International Forum (INOUE, Tohru, sixth CBSR director). Furthermore, the Division of Cellular and Molecular Toxicology has taken a leading role in promoting the large-scale Toxicogenomics Project investigating chemical substances. Because of this project, data on 140 chemical substances and 650 million genes in mice and 200 compounds and 520 million genes in rats have been collected. In the future, a basic technology for safety prediction assessment in humans will be developed utilizing AI through the above databases as well as specialized findings in safety assessment. For verifying and utilizing such technology in actual research, the Division of Risk Assessment of CBSR has taken a leading role by cooperating with other divisions within CBSR as well as the entire NIHS to conduct the “Chemical Substance Safety Big Database Construction and Research and Development into Basic Technology for Safety Prediction in Humans of Medicinal, Food, and Lifestyle Chemical Substances Utilizing Artificial Intelligence.”
During the time the NIHS relocated in 2018, we completed our transfer from our previous building in Yoga to our new building in Tonomachi, Kawasaki. This year of relocation coincided with the 40th anniversary of the establishment of CBSR. We aim to promote the regulatory sciences endorsed by the NIHS by working on new research activities related to the advancement of science and technology, while maintaining steady work in research that must continue as a national research facility.
The Chronological CBSR Directors
| 1. | IKEDA, Yoshio | 1978.1 ~ 1978.7 |
|---|---|---|
| 2. | OMORI, Yoshihito | 1978.7 ~ 1986.3 |
| 3. | TOBE, Masuo | 1986.4 ~ 1990.2 |
| 4. | HAYASHI, Yuzo | 1990.3 ~ 1995.3 |
| 5. | KUROKAWA,Yuji | 1995.4 ~ 2001.3 |
| 6. | INOUE, Tohru | 2001.7 ~ 2010.3 |
| 7. | NISHIKAWA, Akiyoshi | 2010.4 ~ 2018.3 |
| 9. | HIRABAYASHI, Yoko | 2018.4 ~ |


