RESEARCH (Section3)
1. Nano-titanium dioxide with a primary particle size of 6 nm showed no toxic effects when administrated orally to rats (collaboration with Section2)
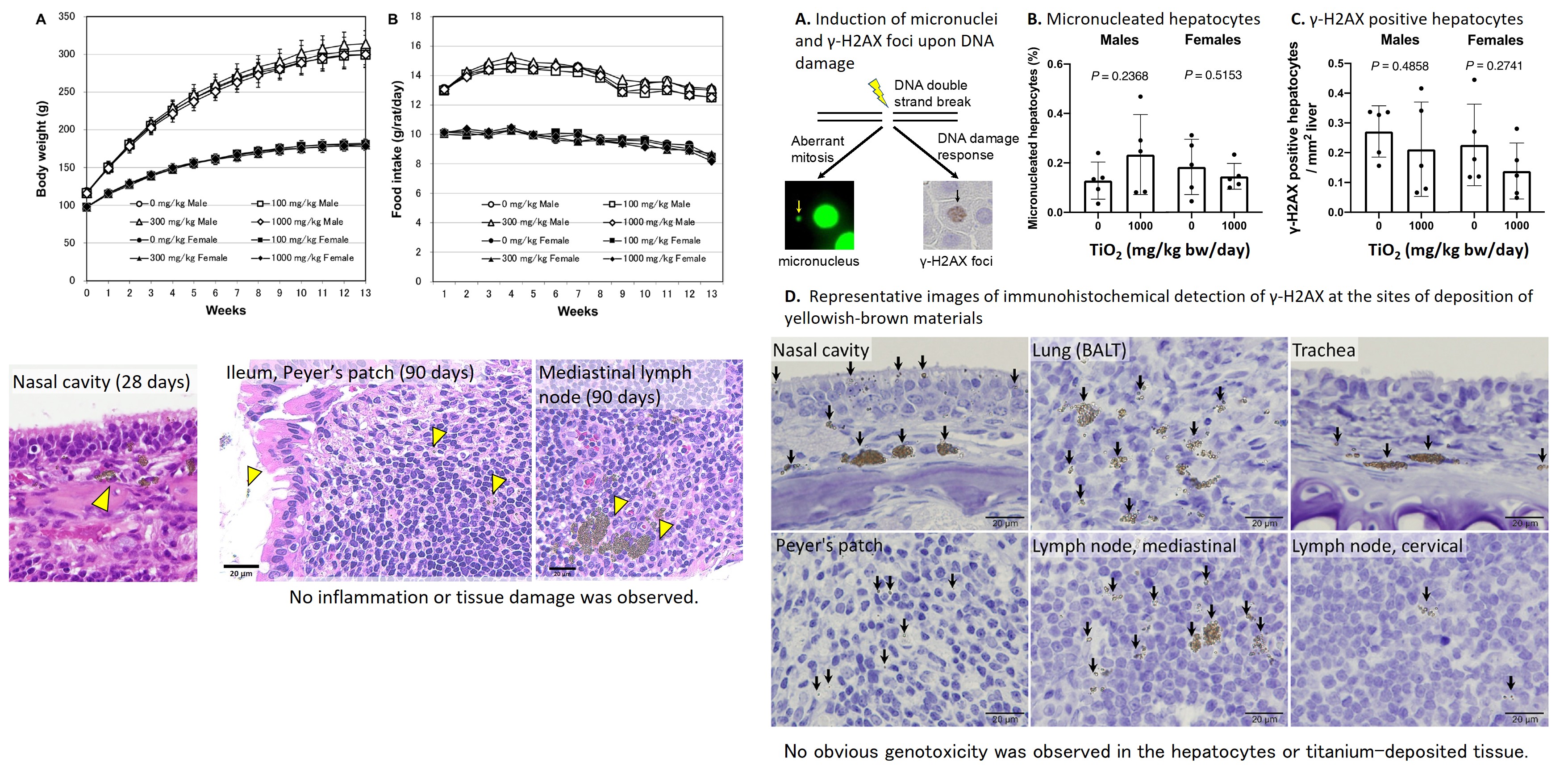
In a 90-day repeated oral administration of the smallest diameter anatase titanium dioxide available in Japan, no toxic effects were observed in blood biochemistry tests, pathological histology tests, etc., up to a dose of 1000 mg/kg body weight/day. Deposition of titanium dioxide aggregates was observed in the Peyer's patches of the small intestine, but was not accompanied by inflammation or tissue damage. In addition, no micronucleus formation in hepatocytes or increase in γ-H2AX-positive DNA damaged cells was observed in the titanium deposition sites of hepatocytes or Peyer's patches. (Part. Fibre. Toxicol., 20: 23, 2023)
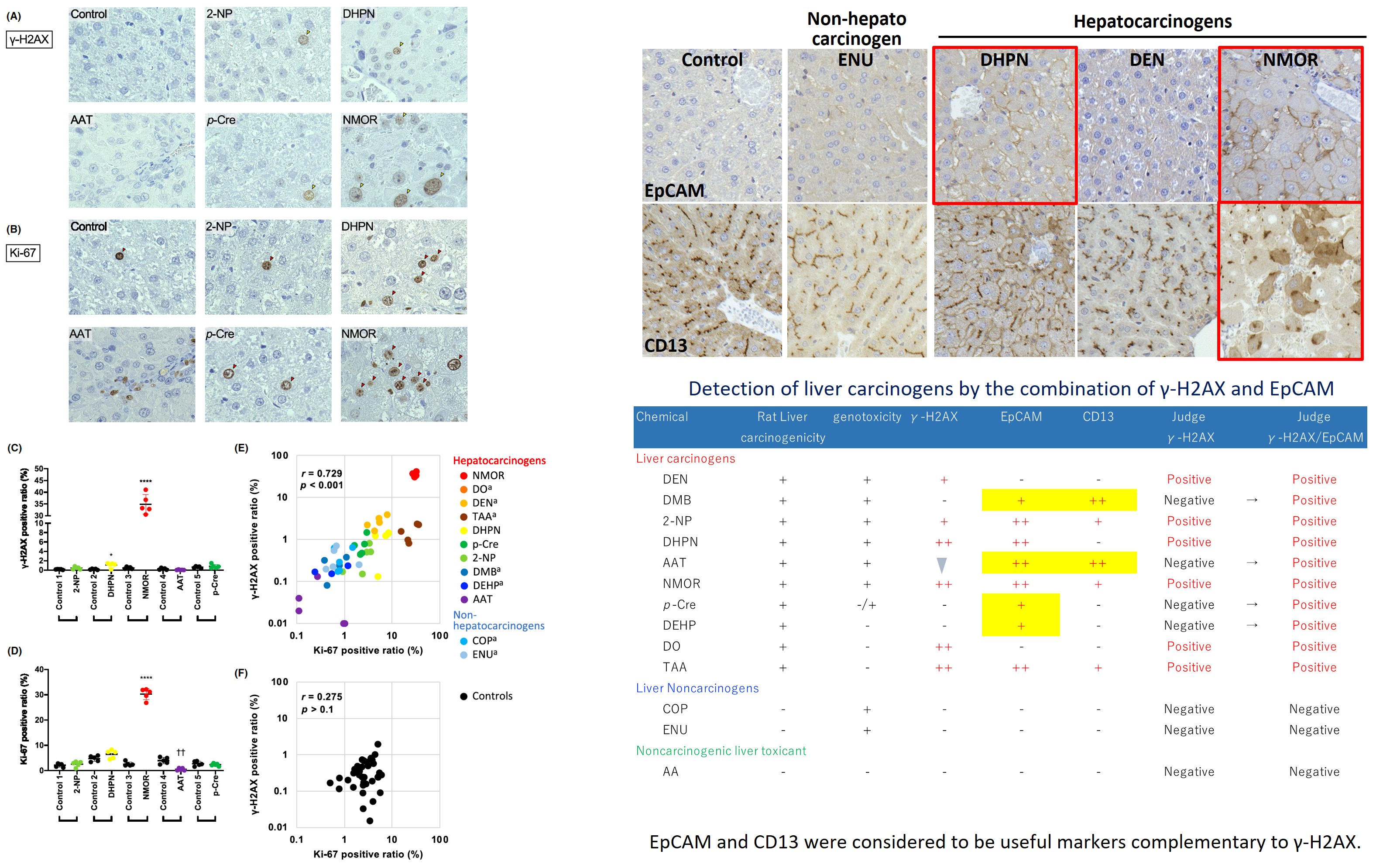
2. Development of an early evaluation method for liver carcinogenicity in rats (collaboration with Section2)
Histopathological evaluation of liver carcinogenicity was performed using specimens from 28-day repeated dose toxicity studies. Since the detection rate of the DNA damage marker γ-H2AX in the liver was approximately 60%, hepatic stem cell markers were also evaluated as complementary markers. (J Toxicol Sci., 48: 323-332, 2023; Cancer Sci., 114: 4763-4769, 2023)
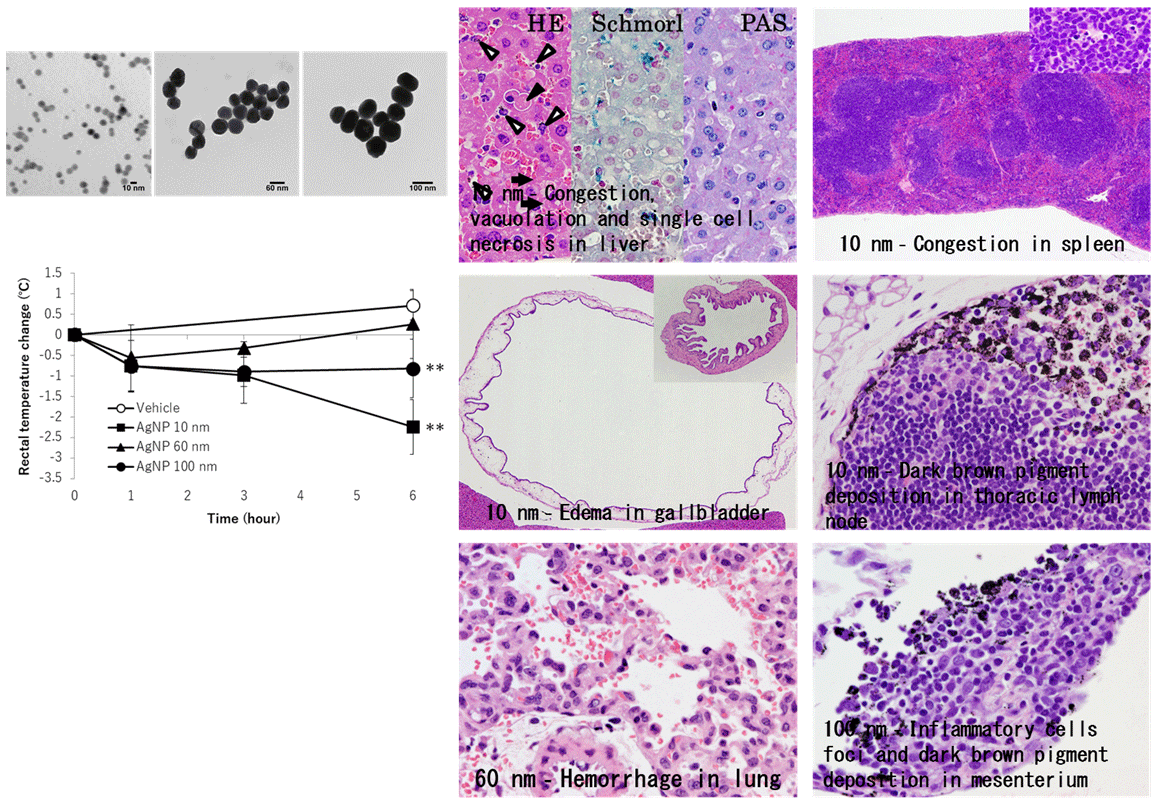
3. Size-dependent acute toxicity of silver nanoparticles
Our results suggested that smaller silver nanoparticles (AgNP) had higher acute toxicity in intraperitoneally administered mice. Now we are studying the mechanisms. (J. Toxicol. Pathol., 31:73-80, 2018)
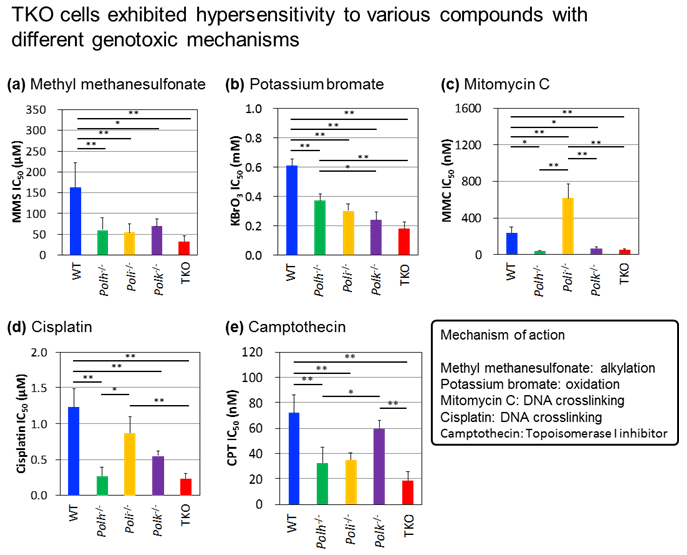
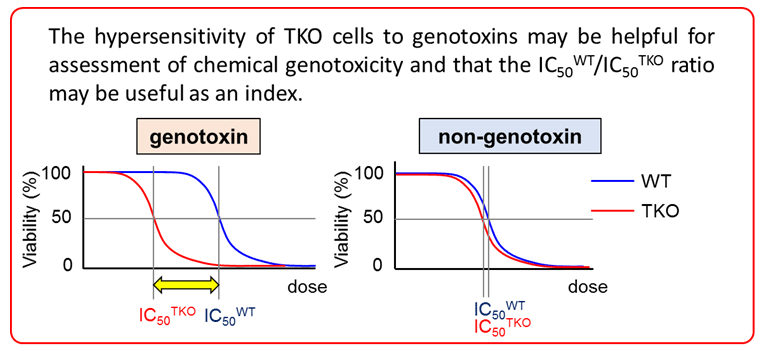
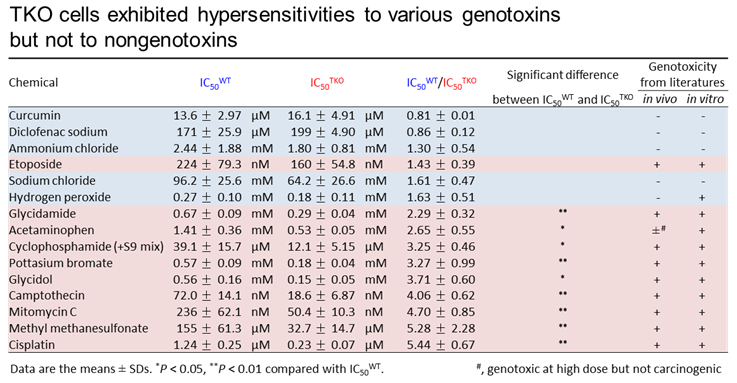
4. Study of Novel genotoxity test using Triple knock-out (TKO) cells defective for translesion DNA polymerases η, ι and κ (DNA Repair, 61:76-85, 2018)