RESEARCH (Section1)
1. Studies on the mechanism of hepatocarcinogenesis of acetamide in rats
A comprehensive toxicity study using gpt delta rats revealed that the rat hepatocarcinogen acetamide has hepatotoxicity. On the other hand, no changes in mutation frequency were observed in the liver, suggesting that lack of mutagenicity in the hepatocarcinogenic process. (Toxicol.Sci., 177:431-440, 2020)
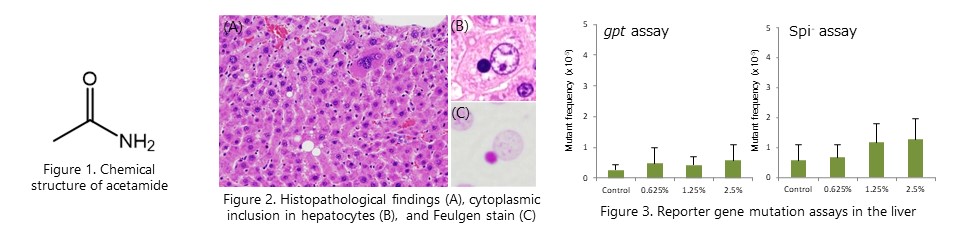
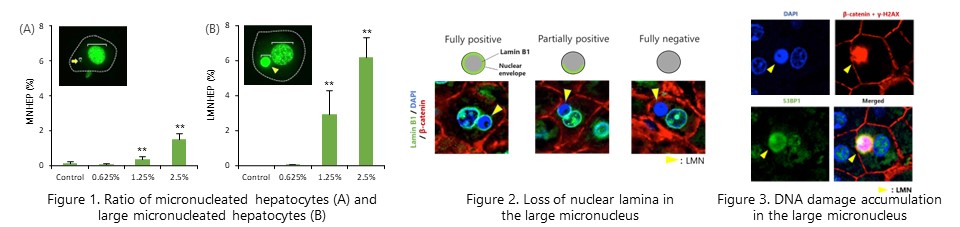
Liver micronucleus assay revealed that the rat hepatocarcinogen acetamide induces chromosomal aberrations specifically in the liver. We also found that large micronuclei, histopathologically observed as cytoplasmic inclusions, exhibit loss of nuclear envelope-related proteins and accumulation of DNA damage. (Arch. Toxicol., 95: 2851-2865, 2021)
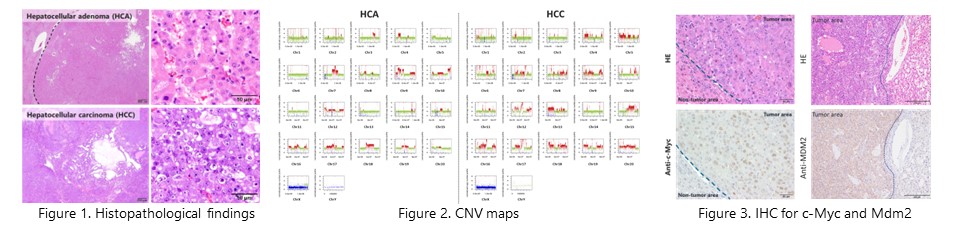
Whole genome analysis using next-generation sequencing revealed that random and large copy number mutations occur in the chromosomes of acetamide-induced tumors. In addition, copy number gains of the oncogenes c-Myc and Mdm2 were frequently observed. These results suggest that chromothripsis-like chromosome rearrangement by large micronuclei contributes to acetamide-induced hepatocarcinogenesis. (Cancer. Sci., 2024, in press) NEW
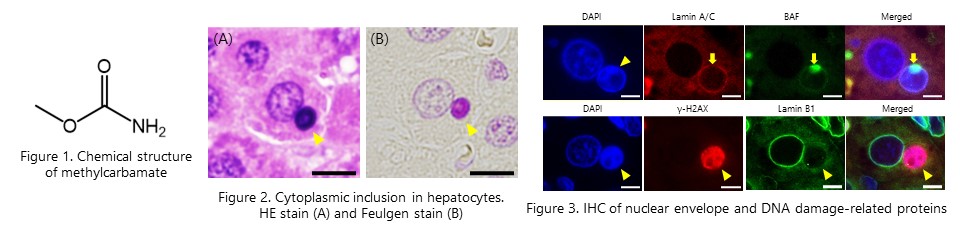
Methylcarbamate, which is structurally similar to acetamide, induced similar large micronuclei in rat liver. The loss of nuclear membrane component proteins and accumulation of DNA damage observed in the large micronuclei suggested that large micronuclei contribute to hepatocarcinogenicity of methylcarbamte. (Toxcol. Sci., 198(1): 40-49, 2024) NEW
2. Studies on the mechanism of renal carcinogenesis of madder color (Application of MSI in toxicologic pathology)
Reporter gene mutation assay and comprehensive DNA adduct analysis using gpt delta rats revealed that the DNA adduct caused by lucidin-O-primeveroside, a component of madder color, is responsible for its carcinogenicity. (Chem. Res. Toxicol., 86:1112-1118, 2012; Anal. Bioanal. Chem., 406:2467-2475, 2014)
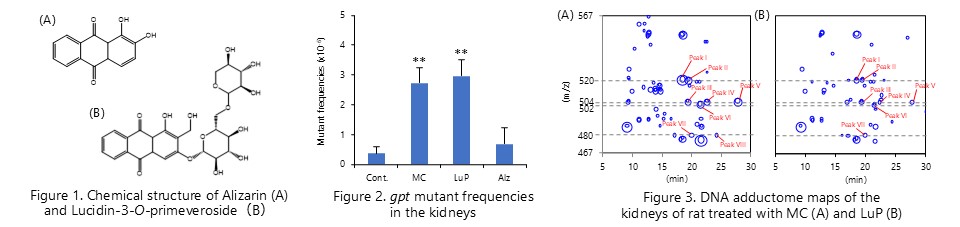
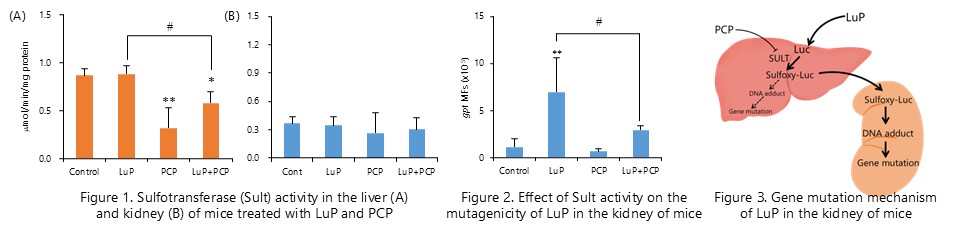
Lucidine-O-primeveroside, a component of madder color, is metabolically activated by hepatic sulfotransferase, causing DNA adduct and gene mutation in the kidney. (J. Appl. Toxicol., 39:650-657, 2019)
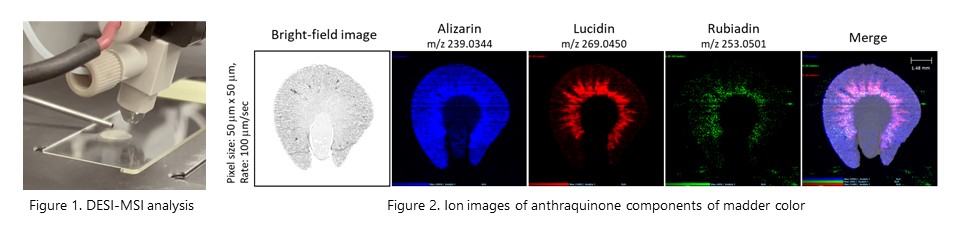
Imaging mass spectrometry using desorption electrospray ionization (DESI-MSI) revealed that several constituents with anthraquinone structure showed different distribution patterns in rat kidney after administration of madder color. Furthermore, we found that genotoxic components, lucidine and rubiadine, are specifically distributed in the outer stripe outer medulla (OSOM), which is the carcinogenic target site of madder color. (Food Chem. Toxicol., 161: 112851, 2022)
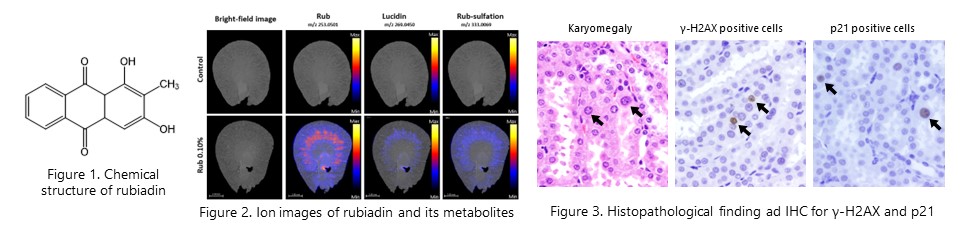
DESI-MSI revealed that rubiadin and its metabolites are distributed in the OSOM in the kidney of rats after repeated 28-day administration of rubiadin. We also showed that distributed rubiadin and its metabolites induce site-specific DNA damage and gene mutation. (Arch. Toxicol., 97(12): 3273-3283, 2023)
3. Studies on the mechanism of hepatocarcinogenesis of alkenylbenzenes
Cell proliferation caused by serine/threonine phosphatase 2A (PP2A) phosphorylation is crucial event for mutagenicity of estragole, in addition to the formation of specific DNA adducts. (Toxicol. Appl. Phramacol., 336: 75-83, 2017)
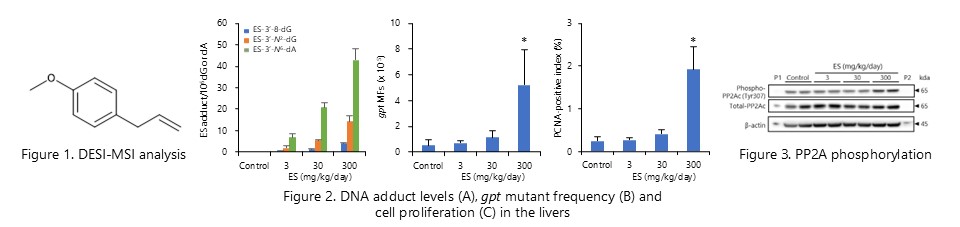
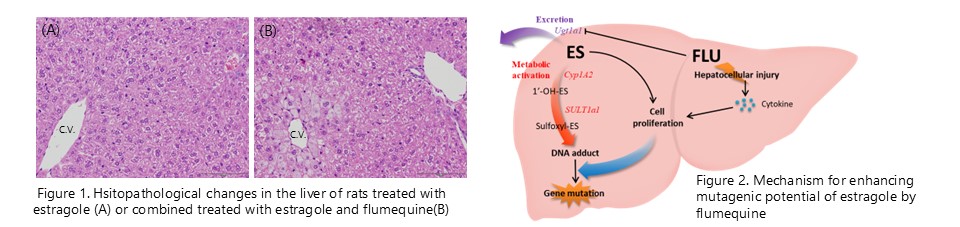
The mutagenicity of estragole is enhanced by flumequine-induced changes in the intracellular microenvironment. (Food Chem. Toxicol., 129:144-152, 2019)
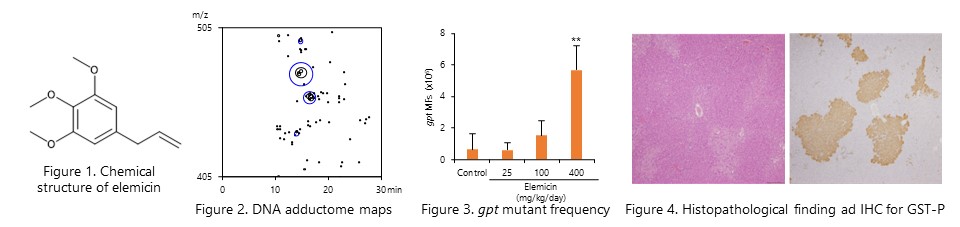
The in vivo genotoxicity of elemicin, a alkenylbenzene compound, was evaluated using gpt delta rats. DNA adduct formation and increased mutant frequency were observed in the liver of both male and female rats. Furthermore, an increase in GST-P positive foci, a marker of liver preneoplastic lesions, was observed, suggesting that elemicin is a genotoxic hepatocarcinogen in rats. (Food Chem. Toxicol, 179: 113965, 2023.)
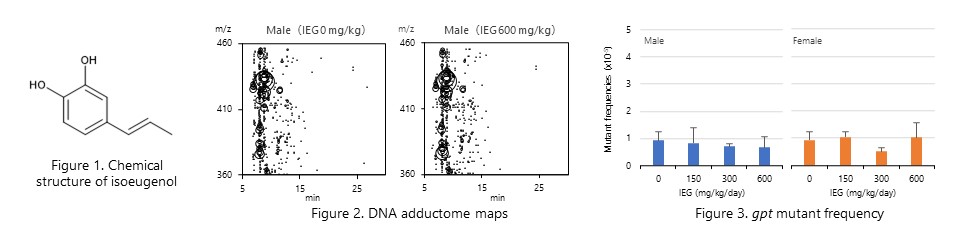
Isoeugenol, which is hepatocarcinogenic in male mice, was evaluated for in vivo genotoxicity using gpt delta mice. The results showed that neither DNA adduct formation nor increased mutant frequency was observed in the livers of both male and female mice, suggesting that hepatocarcinogenesis in male mice is caused by a non-genotoxic mechanism. (Jpn. J. Food Chem. Safety, 30: 9-22, 2023.)
4. Study on the mechanism of gene mutation in chemical carcinogenesis
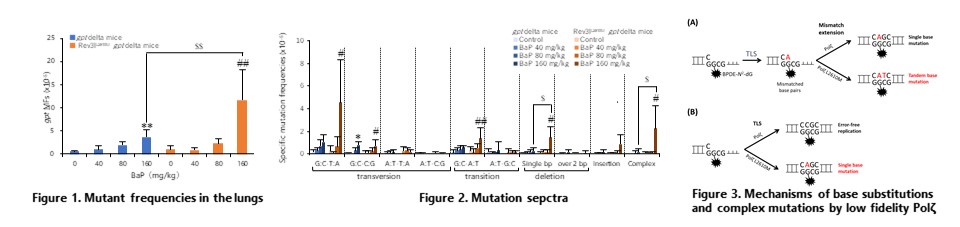
Rev3lL2610M gpt delta mice expressing DNA polymerase zeta (Polz) with low replication fidelity revealed that Polz performs translesion synthesis (TLS) and mismatch extension to benzo[a]pyrene-induced DNA damage. (Mutagenesis, 47: 44-52, 2021)
5. Study on molecular pathological features of liver proliferative lesions in mice
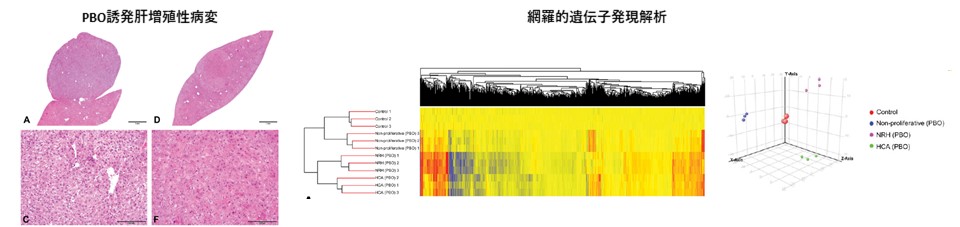
Complementary DNA microarray analysis revealed that the proliferative lesions, nodular hepatocellular hyperplasia and hepatocellular adenoma, observed in the mouse liver after long-term administration of piperonyl butoxide (PBO) exhibited different molecular pathological features. (Toxicol. Pathol., 47: 44-52, 2019)
6. Study on the mechanims of carcinogenesis of acrylamide
We have shown that gene mutations caused by 7-GA-Gua depurination contribute to the process of lung carcinogenesis of acrylamide in mice. (Mutagenesis, 30:227-235, 2015)
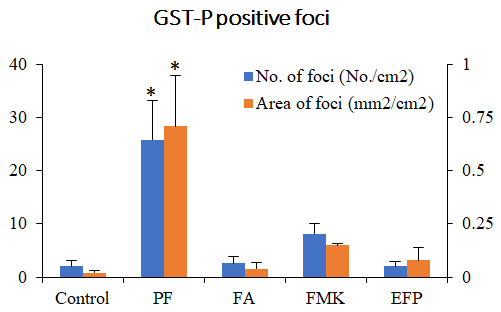
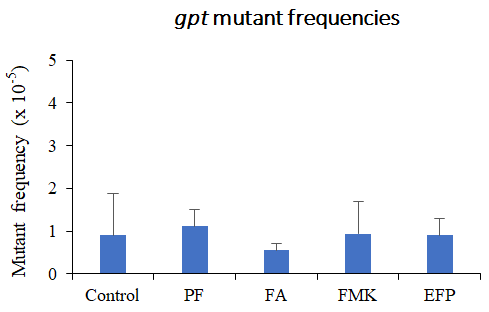
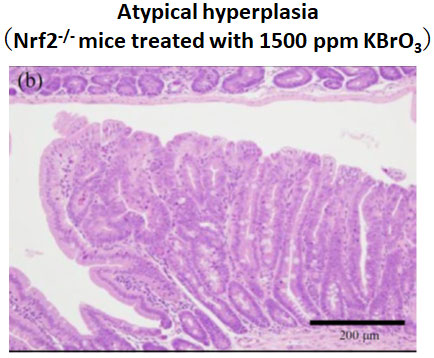
We examined Risk assessment of colorectal cancer in SNPs in NRF2 using KBrO3 treated Nrf2 deficient mice. (Cancer Med., 5:1228-1238, 2016) Our new method showed hepatocarcinogenicity of PF and FMK in rats but not genotoxicity.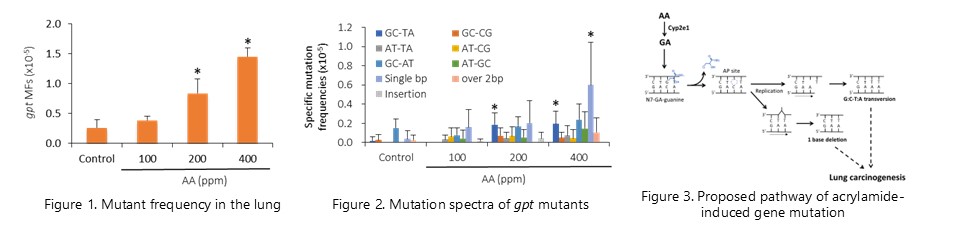
7. Effects of Nrf2 silencing on oxidative stress-associated intestinal carcinogenesis in mice
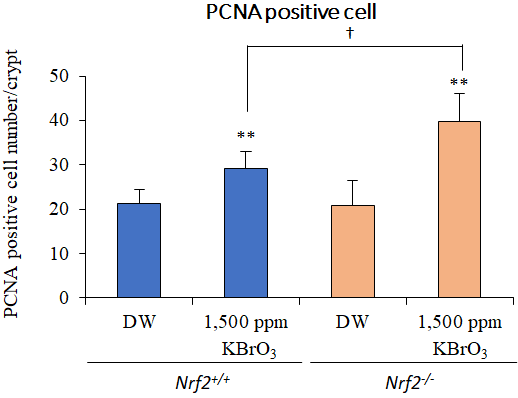
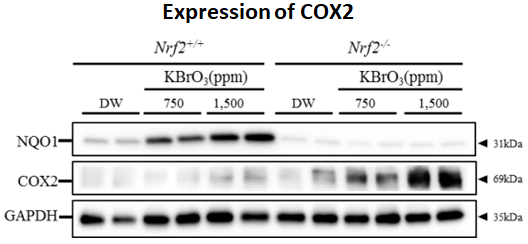
8. Comprehensive toxicity study of flavor agent using a medium-term animal model (GPG) with gpt delta rats.