|
The division have investigated the effects of chemicals including medicines on central nervous system, cardiovascular system and metabolism pharmacologically. In particular, innovation such as human iPS cell technology are used to make more predictive testing methods in human. The division has engaged in development and evaluation of safety testing methods, in establishment of various guidelines related to safety and efficacy, and in committees and conferences for regulation of chemicals as experts. |
|
|
|
|
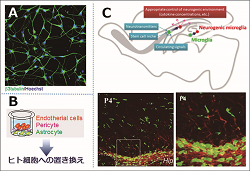
The division are attempting to establish assessment systems to detect adverse effects of new drugs and chemical compounds in the CNS. A: human iPS-derived neurons; B: in vitro BBB model; C: neurogenic microglia in SVZ. |
|
|
|
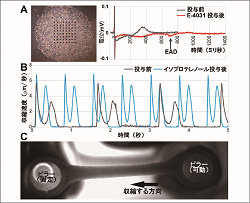
Drug evaluation methods using human cell models, such as human iPS cell derived cardiomyocytes and organoids, has been developed. A: Human iPS cells-derived monolayer sheet on MEA system and drug-induced proarrhythmia; B: Development of image-based cardiac contractility; C: Drug evaluation using three-dimensional model. |
|
|
|
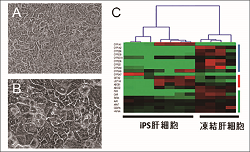
Human hepatocytes which are used for drug metabolism study are evaluated from various aspects. Toxicokinetics is also examined. A, cryo-preserved human hepatocyte: B, human iPS-cell derived hepatocyte: C, evaluation of different kinds of hepatocytes based on gene expression pattern. |
|