|
There are so many kinds of medical devices used clinically used, such as scalpels, cardiac pacemakers, X-ray CT, software and so on. And they are made of different kinds of various materials. The division conducts researches on evaluation mainly for characteristics and safety of various medical devices and their materials for ensuring safety of patients and users. The results and knowledge obtained in the activities are utilized in the drafting of the standards for marketing approval of medical devices. In addition, the division intends to international standardization of evaluation methods/systems for medical devices developed in Japan through industry-government-academia collaboration.
|
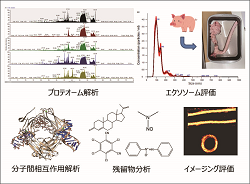
Safety, function and compatibility of innovative medical materials, including biomaterials, are evaluated by various analyses.
|
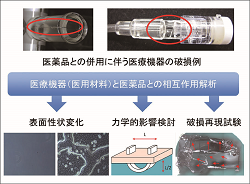
Hazard identification and risk analysis utilizing various methods are performed based on clinical failures caused in medical devices. |
|
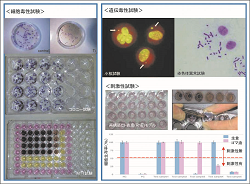
Researches for development and standardization of biological safety evaluation systems for medical devices and materials are conducted.
|
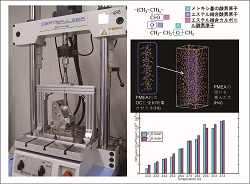
Mechanical properties of implantable materials for medical devices are evaluated. The pilot in silico study on their biocompatibility is also evaluated. |
|