|
In order to ensure the quality, efficacy and safety of synthetic drugs, research activities of the division are focused on the following areas: 1) characterization of physicochemical properties and stability of active pharmaceutical ingredients (APIs), 2) quality management system in the product development, production, and distribution, 3) analysis of formulation composition, 4) bioequivalence tests and alternative methods, 5) pharmaceutical properties and performance of formulations including novel administration route and/or drug delivery system (DDS) products. The results are applied to the revision of general tests and monographs in Japanese Pharmacopoeia and setting new quality-related guidelines. Official tests and precise quality evaluation of pharmaceutical products on the market are also performed to ascertain and improve their quality.
|

Dissolution of drug from a tablet is measured in a simulated digestive fluid in a beaker to investigate the relation with drug absorption. |
|
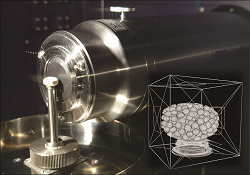
Evaluation of stability and performance of pharmaceuticals using X-ray CT with spatial resolution of hundreds of nanometers. |
|
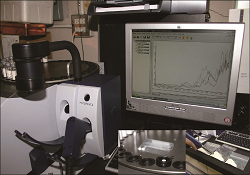
Pharmaceutical active ingredients, excipients or water content of products are characterized using rapid and non destructive near infrared spectroscopy. |
|
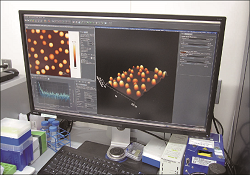
The relationship between the physicochemical properties of nano-sized drug formulations and product performance is studied using state-of-the-art technology. |
|