Our department consists of the First, Second, and Third Sections, and conducts research on the molecular structure and chemical reactivity of chemical substances using organic chemistry methods, as well as research on elucidating the mechanisms of action of harmful substances, with the aim of clarifying the efficacy and safety of various chemical substances including pharmaceuticals.
In addition, for the purpose of developing new pharmaceuticals and various useful substances, we conduct research on the synthesis and reactivity of novel compounds with biological activity, research on structure-activity relationships, and research on structural analysis and elucidation of reactivity of compounds using the latest instrumental analysis methods and computational chemistry methods.
Furthermore, we conduct organic chemistry tests and other tests, inspections, experimental production, and necessary research related to the efficacy, safety, synthesis, structure, and composition of radiopharmaceuticals.
First Section
The first section conducts organic chemical analyses on the synthesis, structure, and composition of chemicals relevant to daily applications and the associated research. In particular, we conduct research on the design and synthesis of compounds with physiological activity and on the quality evaluation of pharmaceuticals.
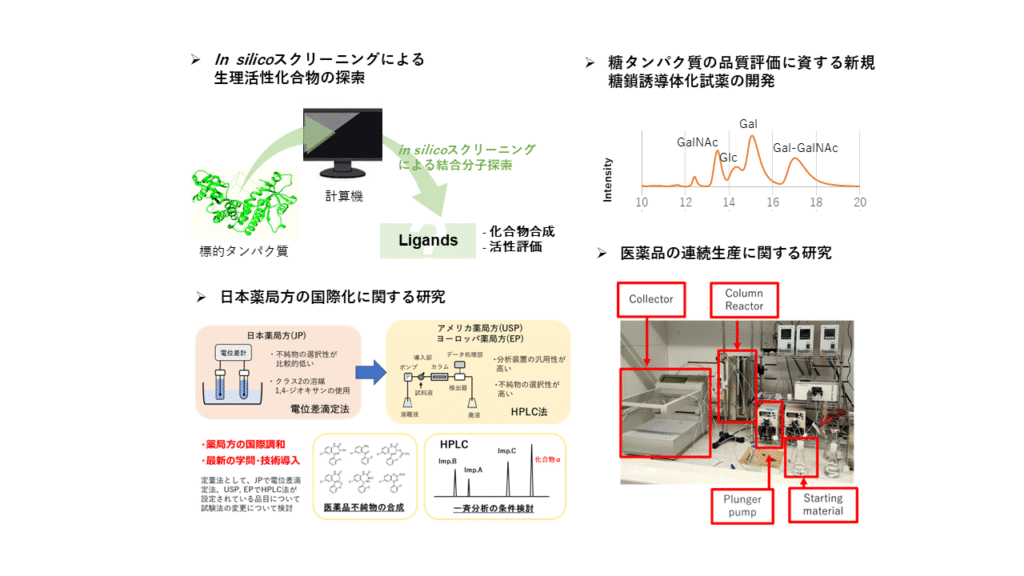
- Design and synthesis of bioactive substances using in silico technology
- Development of novel fluorescent reagents for glycol proteins
- Research on continuous manufacturing of active pharmaceutical ingredients
- Research on the Harmonization of the Japanese Pharmacopoeia
Second Section
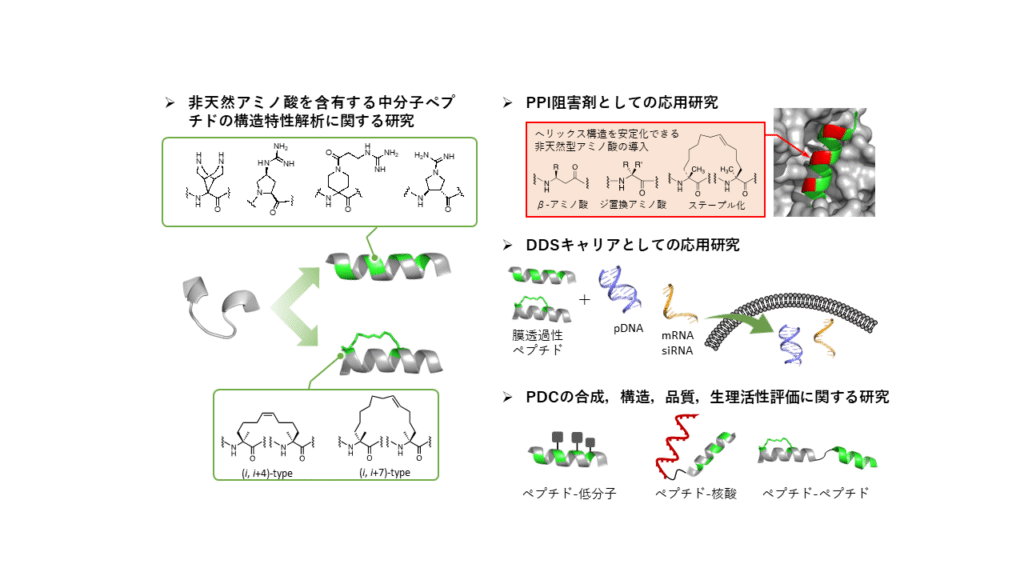
The second sections conducts organic chemical analyses on the synthesis, structure, and composition of chemicals relevant to daily applications and the associated research. Specifically, investigations are carried out to facilitate the efficient development of medium-sized peptide drugs, involving the following:
- Research on the structural characterization of medium-sized peptides containing non-natural amino acids.
- Research on the medium-sized peptides as inhibitors of protein-protein interactions (Protein-Protein Interactions, PPI).
- Research on the medium-sized peptides as drug delivery system (DDS) carriers.
- Research on the synthesis, structure, quality, and bioactivity evaluation of peptide drug conjugates (PDC).
Third Section
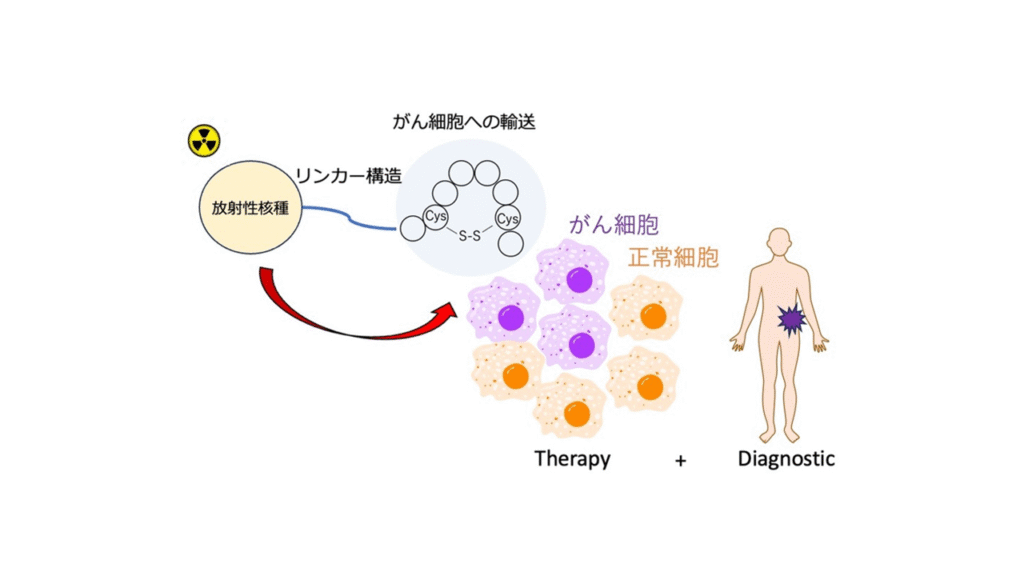
Research to Ensure the Quality and Safety of Radiopharmaceuticals
Radiopharmaceuticals are drugs that contain radioactive isotopes. The radiation from radiopharmaceuticals can be used both to treat disease and to locate and image disease in the body. Combining radiopharmaceuticals used for therapy and diagnostic imaging, a technique known as theranostics, will enable more precise targeting and treatment of disease. While radiopharmaceuticals have the potential to provide excellent therapeutic effects, they may also cause harmful side effects. As a result, accurate assessment of their quality and safety is vital. The third section is conducting research to ensure the quality and safety of radiopharmaceuticals, including the evaluation of impurities in the manufacturing process.
Radiopharmaceutical-Related Guidelines
- Guideline on Clinical Evaluation Methods for Diagnostic Radiopharmaceuticals
- Guideline on Non-clinical and Clinical Trial Design for Therapeutic Radiopharmaceuticals
- Question and Answer (Q&A) Collection on Guideline for Non-clinical and Clinical Trial Design of Therapeutic Radiopharmaceuticals
- Results of Public Comments on "Guideline on Non-clinical and Clinical Trial Design for Therapeutic Radiopharmaceuticals (Draft)"
Joint Research with Related Departments
- Research on Japanese Pharmacopoeia Database for Advanced and Internationalized Standards
- Development of Test Methods for Japanese Pharmacopoeia Revision to Ensure Pharmaceutical Quality
- Research on Formation, Persistence, and Reduction of Pharmaceutical Impurities
- Research on Collection of Analytical Information on Abused Drugs such as Dangerous Drugs and Prediction of Their Harmful Effects
- Research on Supply of Quantitative Reference Standards for Existing Additives by Chemical Synthesis
- Development and Standardization of Next-Generation Safety Evaluation Methods and Acquisition of Foundational Data for Innovative Pharmaceutical Development
- Organic Chemistry Research Contributing to the Development of Protein Degradation Therapeutics
- Investigation of Toxicological Information on Chemical Substances for the Positive List System of Containers and Packaging



